Hydrogen is considered an energy carrier capable of transporting and storing energy for subsequent use in a wide range of applications, from electricity production to transportation and industry. The most common method for hydrogen production today is natural gas reforming, which utilizes methane obtained from a fossil fuel like natural gas, releasing carbon dioxide as a byproduct. This is known as gray hydrogen, whose production is polluting. However, hydrogen can also be obtained through water electrolysis using renewable energy, contributing to the transition towards a cleaner and more sustainable energy system. This process involves splitting water molecules into oxygen and hydrogen using electricity generated from renewable sources such as solar or wind power. Green hydrogen production offers a clean and carbon-free alternative, contrasting with traditional fossil fuel-based production methods.
One of the most significant advantages of green hydrogen is its ability to complement intermittent renewable energies, such as solar photovoltaic and wind technology, helping to improve their manageability. During periods of high energy generation, excess renewable electricity can power electrolyzers, where water electrolysis occurs to produce hydrogen, acting as an energy storage mechanism. This hydrogen can then be used when energy demand is high or renewable generation is low, contributing to ensuring a more stable and reliable energy supply.
Green hydrogen has a wide range of applications and can be used as a fuel for vehicles, thanks to fuel cell technology, providing a clean and efficient alternative to internal combustion engines. Additionally, green hydrogen can be used as a raw material in the production of chemicals, contributing to the decarbonization of the chemical industry and reducing its environmental footprint.
Research in the field of green hydrogen focuses on reducing costs associated with its production, storage, transportation, and application. The challenges in this discipline range from improving the efficiency of the electrolyzer —the central element of the system responsible for electrolysis— to optimizing the coupling between the electrolyzer and renewable technologies, developing safe and efficient storage systems, or implementing low-cost transportation means. Regarding the electrolyzer, three main technologies stand out:
- Proton exchange membrane (PEM) electrolysis, where a membrane that allows the passage of protons while blocking that of electrons is used as an electrolyte. It achieves high efficiency, with a rapid response to power fluctuations, facilitating its integration with renewables. It is a versatile technology that is easily scalable, capable of operating at low temperatures and high pressures. Its major drawback is the scarcity and high cost of some of the materials used, such as platinum or cerium dioxide.
- Alkaline electrolysis generally employs a potassium hydroxide solution as an electrolyte. It is a mature technology that has been commercially used for decades, known for its simplicity and reliability. However, it is somewhat less efficient and flexible, with greater inertia, responding less effectively to transient regimes. Additionally, it uses large amounts of corrosive substances that require maintenance.
- Solid oxide electrolyzers (SOEC) operate at higher temperatures, typically between 700°C and 1000°C, and employ solid electrolytes such as cerium or zirconium oxides, which conduct oxygen ions at high temperatures. It has the potential to achieve higher efficiencies and utilize residual heat for additional processes, but it is still in a developmental stage and must demonstrate its technical and economic viability.
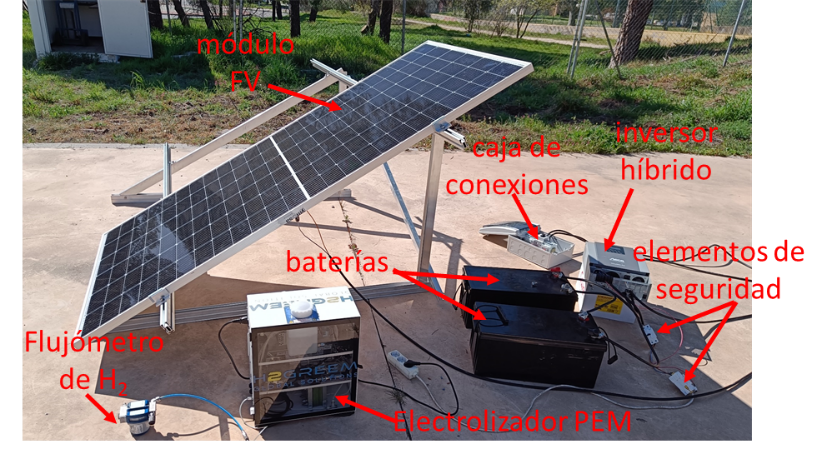
At IES-UPM, we have developed a demonstrator for green hydrogen production based on a PEM electrolyzer, powered by a silicon photovoltaic module, and coupled through a hybrid inverter capable of managing the charge of an electrolytic storage system. This green hydrogen production system, depicted in Figure 1, features electrical characterization elements at each stage and a calibrated hydrogen flowmeter.
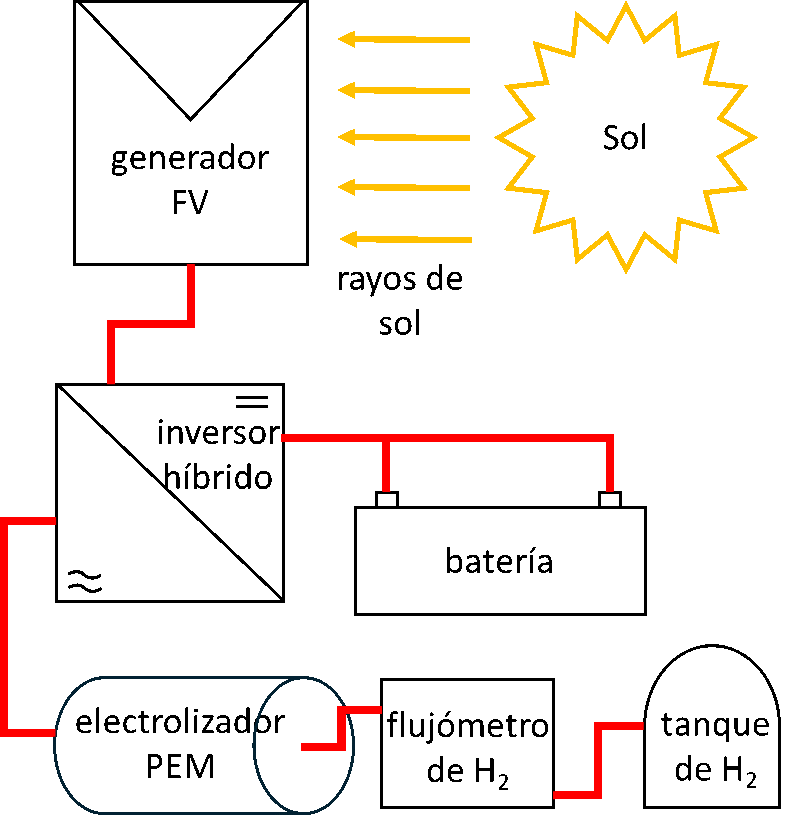
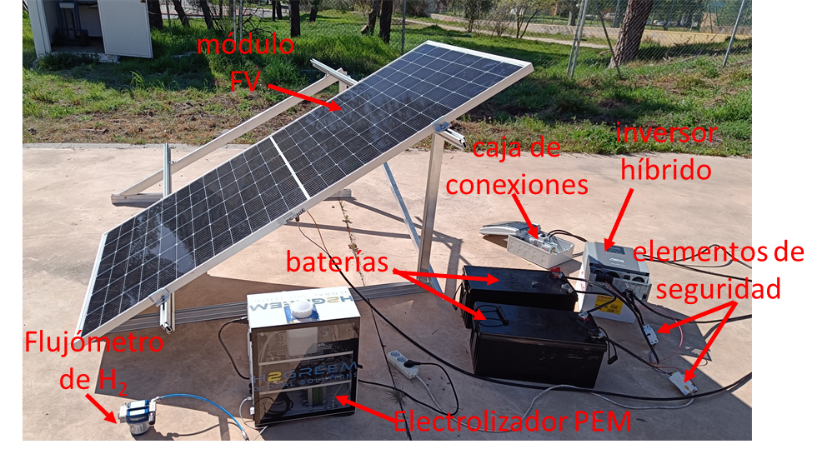
Figure 1. Green hydrogen production system from IES-UPM. (a) Diagram and (b) picture with the main elements of the system.